MEDINEERS PRODUCT DEVELOPMENT SUCCESS STORIES
Case Study 1
Client Situation
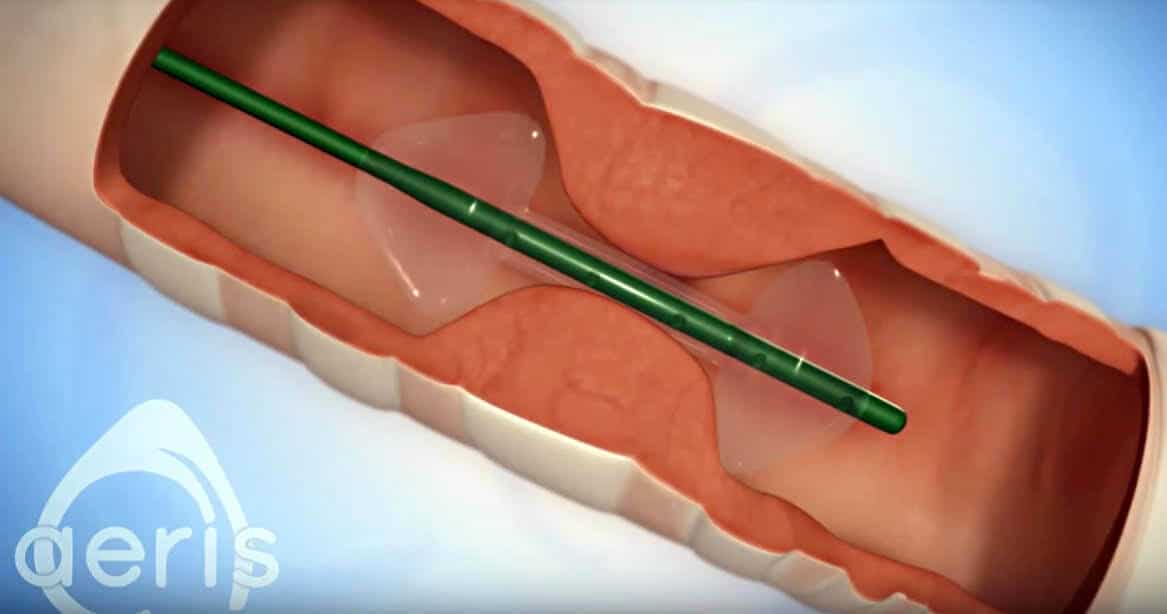
A physician inventor had an idea for novel anesthesia device, but couldn’t find the right team to help get the product beyond the conceptual stage.
Medineers Actions
The Medineers team presented a fast-track plan to engineer and manufacture the anesthesia product. They quickly designed the product, built prototypes, went into pilot production and ramped up the commercialization levels at a contract manufacturer. The inventor and the Medineers team continued to collaborate, and over time, developed and manufactured additional sizes, accessories and product line extensions of the original device.
Results
The device was launched and achieved profitability within the first 12 months, and the company went on to become a dominant player in the market.
Case Study 2

Client Situation
An early-stage company with limited capital approached Medineers to develop a high quality line of substantially equivalent products targeted to a market niche.
Medineers Actions
The Medineers team worked with the customer to develop and contract manufacture the portfolio of competitively-priced products. The client went on to reinvest profits by launching additional products to grow the portfolio, with Medineers handling the engineering and manufacturing of the entire line.
Results
Working together as a team, this strategy allowed the customer to develop and bring to market a quality portfolio of products at a fraction of the cost normally associated with such work. With Medineer’s speed to market, exceptional quality, and attractive pricing, the customer was able to get traction in a competitive marketplace. By outsourcing manufacturing to Medineers, the client was able to focus on product innovation, sales and marketing
Case Study 3
Client Situation
An inventor approached the Medineers team with an innovative enteral feeding device, and requested assistance in finishing the design and pursuing patents.
Medineers Actions
Medineers team quickly completed the product development and built prototypes for product testing and marketing. As a result of beta testing, Medineers added an important component to the intellectual property that the customer went on to patent. Medineers ramped up the production at an ISO 13485 contract manufacturer and has been the outsourced partner for production for the last fifteen years.
Change Delivered
The inventor capitalized on his intellectual property and sold the feeding device to a leading medical products company. For the last fifteen years, Medineers continues to contract manufacture the device just as in the early days, however with substantially higher volume to meet worldwide product demand. Medineer’s quality record speaks for itself: in fifteen years, not a single device has been rejected by the customer
Case Study 4
Client Situation
A successful medical device distributor saw an unfilled need in his marketplace for a safer balloon catheter intended to perform a medical procedure currently done with an off label device.
Medineers Actions
Medineers team won the opportunity with an offer that was faster than the competition. We quickly completed the product development and built adult and pediatric versions of the product. Medineers obtained the FDA 510K for the product and help build an ISO 13485 quality system for the customer. We came in on time, and under budget.
Change Delivered
Medineers ramped up the production at an ISO 13485 contract manufacturer and the product is now on sale domestically and internationally. The company is now a dominant player in the market.
MEDINEERS SERVICES
DESIGN ENGINEERING
Design engineering and manufacturing of high-quality components and finished medical devices in an FDA registered ISO 13485 facility.
FIND OUT MOREMARKETING STRATEGIES
Provide marketing and sales strategies to help medical device clients increase their revenue and profitability objectives.
FIND OUT MOREMERGERS & ACQUISITIONS
Deliver Merger & Acquisition services including exit strategies for owners, pre-acquisition, due diligence, post-acquisition integration strategies, and growth strategies via acquisition.
FIND OUT MORE